In order to promote public education and public safety, equal justice for all, a better informed citizenry, the rule of law, world trade and world peace, this legal document is hereby made available on a noncommercial basis, as it is the right of all humans to know and speak the laws that govern them.
IS 3025 (Part 56) : 2003
(First Revision)
Second Reprint SEPTEMBER 2008
ICS 13.060.50
© BIS 2003
BUREAU OF INDIAN STANDARDS
MANAK BHAVAN, 9 BAHADUR SHAH ZAFAR MARG
NEW DELHI 110002
June 2003
Price Group 3
iEnvironment Protection and Waste Management Sectional Committee, CHD 32
This Indian Standard (Part 56) (First Revision) was adopted by the Bureau of Indian Standards, after the draft finalized by the Environment Protection and Waste Management Sectional Committee, had been approved by the Chemical Division Council.
Pollution caused by substances, on which biotic and abiotic agencies of decomposition are ineffective, is a unique type of pollution. Toxic trace elements and heavy metals come under the category of non-degradable pollutants. The problem caused by these elements is in fact due to their concentration in the environment in the bio-available state and above a certain concentration become harmful to the living organism.
Selenium is widely distributed at low concentrations in the earth’s crust. Selenium can be present in soil due to the bacteria and fungi that are capable of reducing selenites and selenates to elemental selenium and selenides. Surfaces, ocean and well water contain less than 0.05 mg/l selenium, but spring, irrigation—drainage water may contain up to 1 mg/l selenium. Selenium is an essential trace element and selenium deficiency diseases are well known in veterinary medicine.
Selenium (Se) is an essential trace element for living beings. Earth’s crust contains 0.05-0.09 mg/g of Se. Sea water contains about 0.06-0.12 mg/l of this element while soils on an average may contain 0.1-2.0 ppm of this metalloid. About 1-10 mg of Se is present in fossil fuels per kg and it is from the combustion of these fuels in urban localities that almost 90 percent of this element in ambient air is derived. The Se content of air on an average basis ranges between 0.1-10μg/m3. The availability and toxicity of Se to living systems are dependent upon its chemical form and solubility. In nature and in biological system Se occurs as selenates, selenites, elemental selenium, and selenide. While selenates are rather soluble compounds selenites and elemental selenium are virtually insoluble. Selenium compounds may be bio-transformed in the body by incorporation into amino-acids, proteins or by methylation. Biologic role of Se has been attributed to its incorporation in Se-cystein which goes into the organization of glutathione peroxidase. The enzyme reduces organic peroxides and protects membrane lipids and probably proteins also from oxidant damages.
Industrial exposures to hydrogen selenide produce garlic like breath, dizziness and lassitude. Eyes and nasal irritation may also occur. In experimental animals 10 ppm is fatal. In rabbies 0.01 ml of selenium oxy-chloricle, applied dermally results death. Selenium oxy-chloride is a vasicant. It causes blister formation and destruction of tissues of skin exposed to it. Acute selenium poisoning affects central nervous system which includes nervousness, drowsiness and at times convulsions. Symptoms of chronic inhalation exposures include pallor, coated tongue, gastro-intestinal troubles, nervousness, liver and spleen damage, mucosal irritation and lumber pain. Consumption of 100-1 000 ppm of Se with diet causes ‘blind stagger’ in mammals. It is usually in seleniferous areas only that evidence of chronic Se poisoning is observed. Discoloured or decaying teeth, skin eruptions, gastro-intestinal disorders, lassitude, partial loss of hairs and nails, impairment of vision, weakness in limbs and respiratory failures are the common symptoms. Se has also been considered embryo-toxic and terratogenic element. However, an increased Se content has been correlated with a decreased cancer death rates in humans and apparently it exerts a protective action against some types of cancer. In addition, Se is an antidote to the toxic effects of some metals like. As, Hg, and Cd.
As per IS 10500 : 1991 ‘Drinking water—Specification (first revision)’, the permissible limits for selenium in the drinking water is 0.01 mg/l, Max. Beyond this level the water becomes toxic.
However, in view of the environmentally prevalent nature of Se compounds its levels should be closely monitored to avoid any likelihood of toxicity caused by this element.
In the preparation of this standard, considerable assistance is derived from ISO 9965 : 1993 ‘Water quality determination of selenium – Atomic absorption spectrometric method (hydride technique)’ and Standard Methods for the Examination of Water and Waste Water. 19th Edition - 1995, published by the American Public Health Association, Washington. U.S.A. Atomic absorption spectrometric method in this standard is technically equivalent to the method specified in ISO 9965.
The Committee responsible for the formulation of IS 3025 : 1964 had decided to revise the standard and publish it as separate parts. This standard supersedes 28 of IS 3025 : 1964.
In reporting the result of a test or analysis made in accordance with this standard, if the final value, observed or calculated, is to be rounded off, it shall be done in accordance with IS 2 : 1960 ‘Rules for rounding off numerical values (revised)’.
iiIndian Standard
METHODS OF SAMPLING AND TEST (PHYSICAL AND CHEMICAL) FOR WATER AND WASTEWATER
PART 56 SELENIUM
(First Revision)
This standard (Part 56) prescribes two methods for the determination of selenium:
The standards listed below contain provisions which through reference in this text, constitute provisions of this standard. At the time of publication, the editions indicated were valid. All standards are subject to revision and parties to agreements based on this standard are encouraged to investigate the possibility of applying the most recent editions of the standards.
| IS No. | Title |
|---|---|
| 3025 (Part 1) : 1986 | Methods of sampling and test (physical and chemical) for water and waste water: Part 1 Sampling (first revision) |
| 7022 (Part 1) : 1973 | Glossary of terms relating to water, sewage and industrial effluents, Part 1 |
| 7022 (Part 2) : 1979 | Glossary of terms relating to water, sewage and industrial effluents, Part 2 |
For the purpose of this standard, definitions given in IS 7022 (Part 1) and IS 7022 (Part 2) shall apply.
The sampling and storage shall be done as prescribed in IS 3025 (Part 1). The sampling bottles shall be cleaned thoroughly with dilute nitric acid (6N), prior to the final rinsing with water. The water samples should be collected and stored preferably in polypropylene bottles or chemically resistant glass containers. For the determination of dissolved selenium content, filtration through 0.45 μm membrane filter, at the time of sampling, is required. The analysis of such samples is to be carried out within 24 h of sampling. For preservation, the samples should be acidified with concentrated nitric acid (2 ml of conc nitric acid in 1 litre sample, just to bring down the pH below 2). The acidified samples can be stored for a few days (up to 5 days) in a refrigerator.
Unless specified otherwise, only pure chemicals and selenium free distilled water shall be used in tests.
NOTE—‘Pure chemicals’ shall mean chemicals that do not contain impurities which affect the result of analysis.
This method is specific to determine selenite [ SeO32− Se (IV) ] in aqueous solution. Se (IV) reacts with 2, 3-diaminonaphthalene to produce a brightly coloured and strongly fluorescent piazselenol compound, which is extracted in cyclohexane and measured spectrophotometrically at 480 nm. The optimum pH for formation of the piazselenol complex is approximately 1.5 but should not be above 2.5, because, the rate of formation of the coloured compound is critically dependent on pH above pH 2. Minimum detectable quantity by this method is 10 μg Se/l. This method is applicable in the range of 25 to 2 000 μg/l of selenium. Dissolved Se consists predominantly of selenite designated as Se (IV) and selenate designated as Se (VI). When total Se is desired. Se (VI) is reduced to Se (IV) by digestion with concentrated HCl and the total Se is estimated.
No inorganic compound is known to give a positive interference. Coloured organic compounds extractable by cyclohexane may be encountered, but usually they are absent or removed by suitable treatment. Negative interference results from compounds that reduce the concentration of diaminonaphthalene by oxidizing it. Addition of EDTA eliminates negative interference from up to 2.5 mg Fe2+.
1Spectrophotometer—for use at 480 nm, providing a light path of 1 cm.
Separatory Funnel—250 ml, preferably with a fluorocarbon stopcock.
Centrifuge—with rotor, for 50 ml tubes (optional).
Centrifuge Bottles—with fluorocarbon screw cap.
Stock Selenium Solution—Dissolve 2.190 g of sodium selenite in water containing 10 ml of HCl and dilute to 1 000 ml in a volumetric flask [ 1.0 ml = 1.0 mg of Se(IV)].
Standard Selenium Solution—Dilute 10 ml of the stock selenium solution to 1 000 ml with water in a volumetric flask [ 1.0 ml = 10 μg of Se (IV) ].
Hydrochloric Acid, concentrated and 0.1N.
Ammonium Hydroxide, 1 : 1.
Cyclohexane
2, 3-Diaminonaphthalene (DAN) Solution—Dissolve 200 mg of DAN in 200 ml of 0.1 N HCl. Shake for 5 min. Extract three times with 25 ml portions of cyclohexane, each time retain the aqueous phase and discard the organic phase. Filter into an amber coloured bottle and store in cool, dark place. This reagent is to be used within 8 h.
CAUTION—DAN is toxic. So handle with extreme care.
Hydroxylamine + EDTA Solution (HA-EDTA)—Dissolve 4.5 g Na2EDTA in approximately 450 ml water. Add 12.5 g of hydroxylamine hydrochloride (NH2OH-HCl) and adjust the volume to 500 ml.
pH 1.6 Solution—Adjust the pH of deionized water to 1.6 with concentration HCl
pH 12 Solution—Adjust the pH of deionized water to 12 with KOH.
Pipette out suitable volumes of standard selenium solution ranging from 0 to 10 ml (0 ml for the reagent blank) in 50 ml beakers.
Add 2 ml HA-EDTA solution to each of these beakers. Add about 10 ml of water, and adjust pH to 1.5 ± 0.3 using 0.1 N HCl and 1 : 1 NH4OH and a pH meter. Add 5 ml of DAN solution. Cover the beaker with a watch glass and place in the water bath maintained at 50°C for 30 min.
Cool the solution in the beaker. Transfer quantitatively the contents into a 150 ml separatory funnel. Add 3.0 ml of cyclohexane. Shake vigorously for 5 minutes. Wait for 2 min or until cyclohexane layer becomes well separated. (If the cyclohexane layer is not properly separated, transfer the organic phase to a 50 ml centrifuge tube/bottle. Centrifuge for 5 min at 2 000 rpm for proper separation.) Transfer the organic phase to a 5-ml volumetric flask. Use this directly for absorption measurement. Measure the absorbance at 480 nm using cyclohexane as reference.
Pipette out 10 ml or an appropriate amount of the sample containing 10-100 μg of selenium into a 50-ml beaker (sample filtered through 0.45 μ membrane filter, if no coloured organic compounds extractable by cyclohexane are present, or the sample is treated with ion exchange resin for the removal of organic compounds, as described in 6.5.2.2). Add 2-ml of HA-EDTA solution and about 10 ml of water, if required, and carry out the paizselenol complex formation and extraction process as described earlier (6.5.1.2 and 6.5.1.3). Measure the absorbance of the cyclohexane layer at 480 nm. From the absorbance data determine the micrograms of selenium present in 100 ml of the final solution using the calibration curve.
Thoroughly wash the resin (Amberlite XAD-8 or equivalent) with deionized water, remove the fine resin particles by decanting. Wash three times with pH 12 solution. Place 5 cm of the washed resin in a 0.8 cm ID glass column. Precondition the column (at a rate of 1 ml/min) with 30 ml of pH 12 solution, and 20 ml of pH 1.6 solution. Using HCl and pH meter
2adjust sample pH to 1.6 to 1.8. Pass 50 ml of the sample through the preconditioned column at a rate of 1 ml/min. Discard the first few ml of the eluent and collect all the remaining eluent. Use it for the selenium determination as described in 6.5.2 and 6.5.2.1.
Pipette out 10 ml or an appropriate amount of the sample containing 10-100 μg of selenium into a 150-ml beaker. Measure equal amount of water in another beaker for preparing a reagent blank. Add 10 ml concentrated HCl to both the solutions cover the beakers with watch glasses. Place these beakers in a water bath maintained at 95°C for 60 min. Cool the solution to room temperature and use for selenium determination as described in 6.5.2 and 6.5.2.1 using similarly prepared reagent blank. [ If the sample contains coloured organic compounds extractable by cyclohexane, then the same is treated with ion exchange resin for the colour removal (see 6.5.2.2) prior to the reduction step].
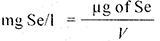
where
V = volume, in ml, of the sample used.
The method is based on the atomic absorption spectrometric measurement of selenium generated by the thermal decomposition of selenium hydride. Under the conditions of this method, only Se (IV) is quantitatively converted to the hydride. To avoid errors in determination, other oxidation states need to be converted to Se (IV) prior to the determination. Se (IV) is reduced to gaseous selenium dihydride (SeH2) by reaction with sodium tetrahydroborate in a hydrochloric acid medium. The absorbance is measured at a wavelength of 196.0 nm.
Table 1 gives details of potential interfering substances which have been present during part of the analytical procedure. The solutions for interference testing were prepared from either solid reagents or concentrated solutions of the reagents, in such a way that 500 ml of the solution contains the stated mass of the other substance and the stated mass of selenium. The solutions were then run through the measurement stage of the method and the results expressed as the effect on the stated mass of selenium.
Assuming the samples containing 250 mg of dried solids are ashed, extracted and diluted to the equivalent of 500 ml, then 100 mg and 250 mg of the other substance used would correspond to the solid samples containing 40 percent and 100 percent of the other substance, respective; and 3.75 μg of Se would correspond to the solid samples containing 15 mg/kg of Se.
Atomic Absorption Spectrometer—Fitted with a hydride system and a suitable radiation source for the determination of selenium, for example, electrodeless discharge lamp or a hollow cathode lamp. A background correction facility may be appropriate.
| Other Substances | Mass of Other Substance | Effect, in μg Se of the Other Substance on 3.75 μg Se |
|---|---|---|
| (1) | (2) | (3) |
| Sodium as chloride | 250 | 0.0 |
| Potassium as chloride | 250 | − 0.1 |
| Calcium as chloride | 250 | 0.0 |
| Magnesium as chloride | 250 | 0.2 |
| Aluminium as sulphate | 250 | 0.2 |
| Lanthanum as chloride | 250 | − 0.3 |
| Borate as sodium salt | 250 | − 0.1 |
| Carbonate as sodium salt | 250 | − 0.2 |
| Nitrate as sodium salt | 250 | − 0.5 |
| Ammonium salt as hydroxide | 250 | 0.0 |
| Phosphate as potassium salt | 250 | 0.2 |
| Sulfate as sodium salt | 250 | 0.0 |
| Fluoride as sodium salt | 250 | − 0.7 |
| Bromide as sodium salt | 100 | 0.2 |
| Iodide as sodium salt | 100 | 0.1 |
| Chromium (III) as chloride | 100 | − 0.2 |
| Manganese (II) as sulphate | 100 | 0.3 |
| Iron (III) as chloride | 250 | 0.5 |
| Cobalt (II) as chloride | 100 | − 1.7 |
| Nickel as sulphate | 100 | − 3.4 |
| Copper (II) as chloride | 250 | − 1.8 |
| Zinc as oxide | 250 | 0.2 |
| Cadmium as chloride | 100 | 0.3 |
| Mercury (II) as chloride | 100 | − 2.9 |
| Tin (II) as chloride | 100 | − 0.4 |
| Lead (II) as acetate | 100 | 0.5 |
| Antimony as sodium tartrate | 250 | − 3.7 |
| Bismuth as nitrate | 100 | − 3.0 |
Gas Supply—with argon or nitrogen.
Glassware—to be cleaned immediately before use with warm, diluted nitric acid (for example, 2 ml/l) and rinsed with water.
Sulphuric Acid—p = 1.84 g/ml.
Hydrochloric Acid—p = 1.16 g/ml.
Hydrogen Peroxide—w (H2O2) = 30 percent (m/m).
Dissolve 1 g of sodium hydroxide in about 20 ml of water. Add 3 g of sodium tetrahydrohorate (NaBH4). Dilute to 100 ml with water. The solution shall be prepared daily.
Place 1.405 3 g of selenium dioxide in a volumetric flask of nominal capacity 1 000 ml. Add 2 g of sodium hydroxide and dissolve in a small quantity of water. Dilute to volume with water (1 ml = 1 mg of Se).
NOTE—Selenium stock solutions are commercially available.
Pipette 10 ml of selenium stock solution into a graduated flask of nominal capacity 1 000 ml. Add 20 ml hydrochloric acid and dilute to volume with water (1 ml ≅ 0.01 mg of Se).
NOTE—This solution is stable for at least 1 week.
Pipette 10 ml of selenium standard solution 1 into a graduated flask of nominal capacity 1 000 ml. Add 20 ml of hydrochloric acid and dilute to volume with water (1 ml = 0.000 1 mg of Se).
NOTE—This solution is stable for at least 1 week.
Pipette 2 ml of hydrochloric acid into a graduated flask of nominal capacity 100 ml, and dilute to volume with water. Treat the blank in exactly the same way as the sample.
Using selenium standard solution 2 (see 7.4.8). prepare at least five calibration solutions covering the expected working range.
For example, for the range 1 μg/l, pipette 1 ml, 3 ml, 5 ml, 8 ml and 10 ml of selenium standard solution 2 (see 7.4.8) into a series of 100 ml one-mark volumetric flasks. To each of these flasks, add 2 ml of hydrochloric acid and dilute to volume with water. These solutions correspond to selenium concentrations of 1 μg/l, 3 μ/l, 5 μg/l, 8 μg/l and 10 μg/1 respectively. The calibration solutions shall be prepared daily.
For the determination of the total selenium content, the samples shall be digested in order to decompose organic selenium compounds. If experience has shown that the selenium will be recovered quantitatively without decomposition, the digestion process (7.5.3.1) may be omitted.
Add 5 ml of sulphuric acid (see 7.4.1) and 5 ml of hydrogen peroxide (see 7.4.3) to the round-bottomed flask (see 7.3.3). Add some boiling beads and connect the flask to an apparatus as shown in Fig. 1. Close the cock. Heat the contents of the flask to boiling and collect the condensate in the condensate reservoir. Continue heating until turbid fumes of sulphuric acid appear. Check the appearance of the sample. If it is turbid and almost colourless, cool and add another 5 ml of hydrogen peroxide and continue boiling as described above. After cooling, return the condensate to the round bottomed flask.
NOTE—Care should be taken to ensure that the sample is never evaporated to complete dryness.
Add 20 ml of hydrochloric acid to the round bottomed flask. Gently boil the mixture under reflux for 15 min with the cock open. If there is no prior digestion and if the sample contains free chlorine, aerate the solution with nitrogen (about 1 l/min) for the same period of time. Cool the samples solution and transfer it quantitatively into a graduated flask of nominal capacity 100 ml. Dilute to volume with water. Treat the blank solution (see 7.5.1) and the calibration solutions (see 7.5.2) in the same way.
Depending on the hydride system used, volumes that are greater or smaller than those described in this subclause may be used. However, the quantity ratios defined shall be maintained. Set all the instrumental parameters of the atomic absorption spectrometer in accordance with the manufacturer’s operating manual (wavelength; 196.0 nm) and optimize the position of the absorption cell in order to obtain a maximum transmission.
4
Fig. 1 Example of Decomposition Apparatus
Measure the solutions in the following order:
Pass a stream of argon or nitrogen through the system and zero the instrument. Introduce, for example, 20 ml of the reduced solution (see 7.5.3) into the reaction vessel. Connect the reaction vessel to the hydride system. Pass argon or nitrogen through the solution until the absorption signal returns to zero. Add about 5 ml of sodium tetrahydroborate solution (see 7.4.5) to the solution and record the signal. Establish the calibration curve using values obtained with the blank and calibration solutions. Repeat the procedure using separate portions of each solution.
5NOTE—It is good practice to check the blank and calibration points from time to time. With unknown samples, it is good practice to check the validity of the method by adding a known volume of selenium, in one sample at least. If recovery tests are not satisfactory, the procedure of standard additions should be used.
Obtain the mass concentration of selenium, in micrograms per litre, in the measuring solution on the basis of the absorbance and the calibration function. All dilution steps shall be taken into account.
An interlaboratory trial, carried out in 1992 with an almost identical method, yielded the results given in Table 2.
| Sample No. | L | n | na percent | x μg/l | X μg/l | σr μg/l | VCr percent | σR μg/l | VCR percent | WFR percent |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 19 | 50 | 0 | 3.0 | 2.92 | 0.52 | 18.0 | 0.19 | 6.5 | 97.4 |
| B | 19 | 42 | 11 | 9.0 | 7.76 | 5 | 11.2 | 1 | 5.7 | 86.2 |
| 0.86 | 0.43 | |||||||||
| 9 | 9 | |||||||||
|
L = number of laboratories, n = number of values. na = percentage of outliers, x = true value. X = total mean, σr = repeatability standard deviation, VCr = repeatability variation coefficient, σR = reproducibility standard deviation, VCR = reproducibility variation coefficient, and WFR = recovery rate. A = drinking water B = wastewater |
||||||||||